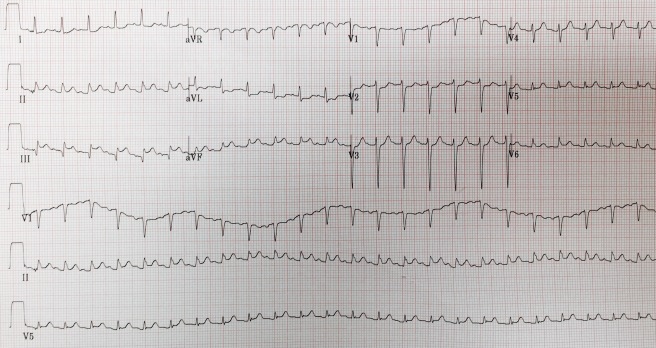
An 85-year-old female presents from her nursing home in the respiratory failure. EMS found the patient in agonal respirations. On arrival to the ED the patient is intubated, heart rate 150, blood pressure 40/palp, and she has palpable femoral pulses. As I run through possible next steps to keep this patient from impending arrest, EMS shows me the EKG. Should we activate the cath lab?
 Continue reading
Continue reading
Month: September 2015
The pupils are fixed and dilated. The pupils are fixed and dilated?!
Written by Zach Adams, OSUEM resident // edited by Michael Barrie, OSUEM Assistant professor
EMS brings in an unconscious man. They are bagging the patient in the hallway of the ED and tell you that they found the patient “down” at home, unresponsive, and with agonal respirations. The patient is obviously altered, unresponsive, and not protecting his airway. You and the team respond rapidly, performing rapid assessment on this undifferentiated patient. Rapid sequence intubation is performed to protect the airway, and you go down your algorithm. The patient was not moving spontaneously, and you’d like to assess pupillary status. But he’s intubated, sedated, and just received etomodate and rocuronium. The pupils appear dilated and unresponsive. But is the pupil exam reliable after a paralytic?
Does D-dimer rule out PE? YES, but…
Written by Zach Adams, OSU EM resident // Edited by Michael Barrie EM Assistant Professor.
Bottom line: D-dimer reliably excludes PE in low/moderate risk patients, however use clinical history/exam to guide pursuing advanced imaging.
An otherwise healthy 23 year-old presents with worsening shortness of breath for the past 7 days. Shortness of breath began suddenly while at rest, has been continuous, and with associated reduced exercise tolerance. Five days ago she was seen in the ED with a negative d-dimer and CXR and discharged with return precautions. Since then, she states that the symptoms have progressed. She says she cannot lie flat. ROS reveals a history of antecedent URI 10 days ago. She is a non-smoker, takes no birth control pills, does not have a personal or family history of DVT or PE, and denies recent prolonged travel. She appears uncomfortable and takes deep inspirations every 3-5 seconds. Her physical exam reveals tachypnea with otherwise normal exam. ECG shows normal sinus rhythm. CXR is normal and repeat d-dimer is negative.
The patient has bounced back with worsening symptoms. I struggled with this clinical question: should we obtain a CT PE to rule out PE despite a negative d-dimer in this low risk patient?
Intern Review – Parapneumonic Effusions
Written by Patrick Sylvester, OSU EM/IM Resident // Edited by Michael Barrie OSU EM Assistant Professor
“Never let the sun set on a…”
During a recent scanning shift performing point of care ultrasounds, we came across an old adage that we had to google to confirm was true: “Never let the sun set on a…” We each inserted our own ending based on the wise words we had heard in medical school. I suppose from the title of this post, you can guess what we ended up talking about—after some googling, that is. While our argument was settled that one was not to let the sun set on a parapneumonic effusion, we were unsure best practice in more ambiguous cases.
Consider this: you’re called to the bedside of a 60 year-old patient who presented with hypotension, subjective fever, and in Afib w/ RVR. A rapid assessment with the ultrasound probe can provide critical information; a subxiphoid view for cardiac contractility and evaluation of pericardial effusion, the IVC for preload assessment. Perhaps you sweep by one of the costodiaphragmatic angles and find something like what’s shown in following video:
In the undifferentiated ill patient, the cause of the pleural effusion is not always clear. Is it secondary to decompensated CHF or is this a parapneumonic effusion? Obviously the clinical history and exam, imaging studies, (and perhaps the other sonographic views we mentioned), labs studies, etc that would lead you to the most likely conclusion. But…
Can bedside thoracic ultrasound diagnose a parapneumonic effusion? Or are we doomed to google Light’s criteria once more and prepare for a rather uncomfortable bedside procedure?
It turns out that there have been a number of studies attempting to correlate the sonographic assessment of an exudative effusion and formal definition by Light’s criteria on thoracentesis. While clinical decision rules have not been studied in large prospective trials, the following are some features of thoracic ultrasound that have been commonly used to classify pleural effusions as transudative or exudative in nature.
| Transudates | Features | Exudate | |
| No septations | Anatomy | Septations | Septations present |
| Anechoic | B-Mode | Echogenicity | Hypoechoic, heterogeneous internal echoes |
| < 3 mm | Calipers | Pleural thickness | >3 mm |
This is consistent with recommendations from 2012 that arose from the International Liaison Committee on Lung Ultrasound (more casually known as the ILC-LUS, of course). Interestingly, of these characteristics, it turns out that echogenicity can be somewhat misleading. While it would appear that the finding of internal echoes and/or hypoechoic fluid is highly suggestive of an exudative effusion, the absence of these findings does not guarantee that it is a transudate. In one study, 14% of anechoic pleural effusions were eventually found to be exudates by pleural fluid analysis. Like many findings on POC ultrasound, finding Hypoechoic, heterogeneous internal echoes is specific for parapneumonic effusions, however not sensitive enough to exclude when the effusion is anechoic.
Based upon our newly found visual Light’s criteria, you might consider that the effusion in the video above is consistent with an exudative process based on the somewhat hypoechoic fluid with heterogenic echoes. In the setting of presumed pneumonia, it might be reasonable to observe these expectantly based on guidelines from both the American College of Chest Physicians (ACCP) and American Thoracic Society (ATS) which describe characteristics of effusions that do not warrant immediate thoracentesis:
- Effusions <10 mm thickness based on decubitus X-ray (surely a common order into today’s ED’s), CT, or thoracic ultrasound.
- Free-flowing effusion, without evidence of loculations
Of course, if an effusion did not fit the criteria mentioned above, it would then be appropriate to perform thoracentesis. And if one of the following characteristics were found, consider the placement of a small (<18 Fr) chest tube for drainage. (Luckily, only about 10% of all parapneumonic effusions will meet this criteria)
Consider placement of small chest tube:
| A | Anatomy | Large (> ½ hemithorax), loculated effusions |
| B | Bacteriology | Positive gram-stain |
| C | Chemistry | pH <7.20 |
In the end, what started as a discussion of “things our attendings said in medical school” turned into a great review of a disease process for which we can do a great deal in the way of work-up quickly at the bedside.
References:
- Sahn SA, Light RW. The sun should never set on a parapneumonic effusion. Chest. 1989;95(5):945-7.
- Muhammad S, Azam R, Owen W, Kamalanathan M, Toma T. A simple score based on ultrasound criteria to distinguish between exudative vs. transudative pleural effusions. European Respiratory Journal. 2014.
- Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-91.
- Sajadieh H, Afzali F, Sajadieh V, Sajadieh A. Ultrasound as an alternative to aspiration for determining the nature of pleural effusion, especially in older people. Ann N Y Acad Sci. 2004;1019:585-92.
- Marcun R, Sustic A. Sonographic evaluation of unexplained pleural exudate: a prospective case series. Wien Klin Wochenschr. 2009;121(9-10):334-8.
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions : an evidence-based guideline. Chest. 2000;118(4):1158-71.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc. 2006;3(1):75-80.
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions : an evidence-based guideline. Chest. 2000;118(4):1158-71.
Intern Review – Can CT Angiography Rule Out Subarachnoid Hemorrhage?

The bottom line – CTA may be able to reasonably exclude SAH when pre-test probability is relatively low and LP is non-diagnostic.
A 55 year old male with PMH significant for HTN and DM presents with new onset worst headache of his life. He is hemodynamicly stable and physical examination is without focal neurologic deficit. You suspect SAH and proceed with non-contrast CT head which is negative. An LP is attempted but fails. What are your options?
Given the significant morbidity and mortality of this condition and up to 10% are misdiagnosed on initial visit to the ED (1, 2), a careful workup is warranted when to rule out SAH.
The sensitivity for detecting SAH with a non-contrast head CT decreased with time:
- <6 hours – 100% (3)
- 6-12 hours – 98%
- 12-24 hours – 93% (4)
- 24 hours to 5 days – <60%
After 10 days, subarachnoid blood is resorbed and the study becomes useless (5). When CT is negative, the next move is an LP to look for RBCs and xanthrochromia in the CSF. CSF xanthrochromia takes about 2-12 hours to develop but can be detected for up to 3 weeks or longer (6, 7). In one study, however, traumatic taps may produce a false positive report if not processed within 2 hours of CSF collection (8).
When the CSF is analysed, the RBC count in the 3rd or 4th tube is used to make the diagnosis. While no specific cutoff exists for the number of RBCs that may constitute a negative tap, a reduction of RBCs by 25% from tubes 1 to 4 is sometimes used. A recent publication does suggestion that less than 2000 RBCs and no xanthochromia is sensitive enough to rule out SAH (12). This method, however, may itself be folly to definitively say no SAH exists, as at least one study has shown this reduction in RBC count may occur when in fact an SAH is present (9). Therefore, only a negative CT and LP combined can reliably rule out the process, a process that that has improved with the newer generation CT scanners (10).
Back to our case. So what do we do when we can’t obtain an LP? In a small study of 116 patients, CT angiography was used in combination with CT and LP for the diagnosis of SAH (11). in this study, CT angiography added diagnostic utility to a negative CT and LP by finding 2 cases that would have been missed by a negative CT and LP alone. So what about just doing CT and CTA?
In a study published in Academic Emergency Medicine in 2010, a mathematical probability model using a pretest probability of 15% or less for SAH (acute-onset headache, non-focal neurological exam) was utilized to determine posttest probability of excluding aneurysmal or AVM related SAH with CT/CTA alone. In this model, combining CT/CTA was able to exclude SAH with a 99% posttest probability. Mathematically, the pretest probability for a negative CT/CTA with LP puts the risk of a missed SAH at less than 1%.
Why not just do CT/CTA then for everyone? For the majority of individuals whom we are considering SAH as a diagnosis, the pretest probability is higher than the aforementioned study for an ED population. Simply relying on a CT/CTA alone therefore puts us a risk of missing some of the lower risk patient population. That said, it is likely a reasonable strategy in those unable to undergo LP or will not consent to the procedure. An alternative in our case might be to perform an fluoroscopic guided LP – as long as the resources are available. Using ultrasound could potentially improve LP success when landmarks are not easily palpated. When these options are not available, ordering a follow up CTA is a reasonable next step. At least then you may be reassured that the inability to get an LP decreases the overall chances of missing something sinister.
Written by Dr. Daniel “Zach” Adams, Intern at OSU EM.
Edited by Dr. Michael Barrie, Assistant Professor at OSU EM.
References:
- Vermeulen MJ, Schull MJ: Missed diagnosis of subarachnoid hemorrhage in the emergency department.Stroke 38: 1216, 2007.
- Kowalski RG, Claassen J, et al: Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA291: 866, 2004.
- Perry JJ, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. 2011; 343:d4277.
- van Gijn J and van Dongen KJ, The time course of aneurysmal haemorrhage on computed tomographs. Neuroradiology. 1982; 23:153-156.
- Al-Shahi R, White PM, et al: Subarachnoid hemorrhage. BMJ 333: 235, 2006.
- Chalmers AH, Kiley M: Detection of xanthochromia in cerebrospinal fluid. Clin Chem 44: 1740, 1998.
- Sidman R, Spitalnic S, et al: Xanthochromia? By what method? A comparison of visual and spectrophotometric xanthochromia. Ann Emerg Med 46: 51, 2005.
- Graves P, Sidman R: Xanthochromia is not pathognomonic for subarachnoid hemorrhage. Acad Emerg Med 11: 131, 2004.
- Heasley DC, Mohamed MA, Yousem DA: Clearing of red blood cells in lumbar puncture does not rule out ruptured aneurysm in patients with suspected subarachnoid hemorrhage but negative head CT findings. Am J Neuroradiol 26: 820, 2005.
- Boesiger BM, Shiber JR. Subarachnoid hemorrhage diagnosis by computed tomography and lumbar puncture: are fifth generation CT scanners better at identifying subarachnoid hemorrhage?. J Emerg Med. 2005 Jul. 29(1):23-7.
- McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med. 2010 Apr;17(4):444-51.
- Perry JJ, et al. Differentiation between traumatic tap and aneurysmal subarachnoid hemorrhage: prospective cohort study. BMJ. 2015 Feb 18;350:h568. [Free open access article]